Cadmium »
PDB 1jv4-1mwr »
1kcq »
Cadmium in PDB 1kcq: Human Gelsolin Domain 2 with A CD2+ Bound
Protein crystallography data
The structure of Human Gelsolin Domain 2 with A CD2+ Bound, PDB code: 1kcq
was solved by
S.L.Kazmirski,
R.L.Isaacson,
C.An,
A.Buckle,
C.M.Johnson,
V.Daggett,
A.R.Fersht,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 22.25 / 1.65 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 96.968, 26.525, 50.294, 90.00, 121.19, 90.00 |
| R / Rfree (%) | 17.7 / 23.3 |
Cadmium Binding Sites:
The binding sites of Cadmium atom in the Human Gelsolin Domain 2 with A CD2+ Bound
(pdb code 1kcq). This binding sites where shown within
5.0 Angstroms radius around Cadmium atom.
In total 5 binding sites of Cadmium where determined in the Human Gelsolin Domain 2 with A CD2+ Bound, PDB code: 1kcq:
Jump to Cadmium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Cadmium where determined in the Human Gelsolin Domain 2 with A CD2+ Bound, PDB code: 1kcq:
Jump to Cadmium binding site number: 1; 2; 3; 4; 5;
Cadmium binding site 1 out of 5 in 1kcq
Go back to
Cadmium binding site 1 out
of 5 in the Human Gelsolin Domain 2 with A CD2+ Bound
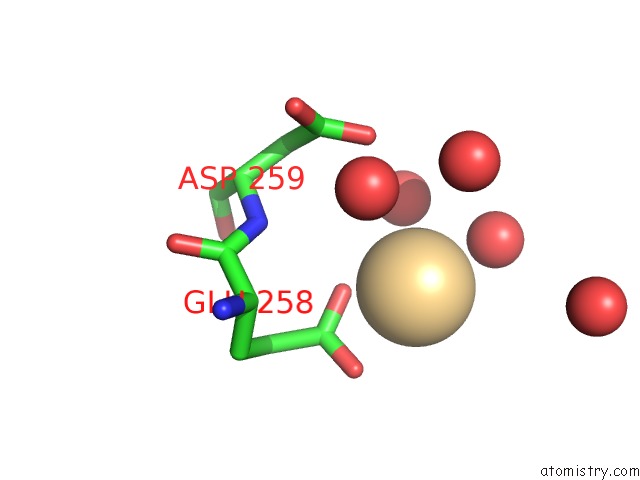
Mono view
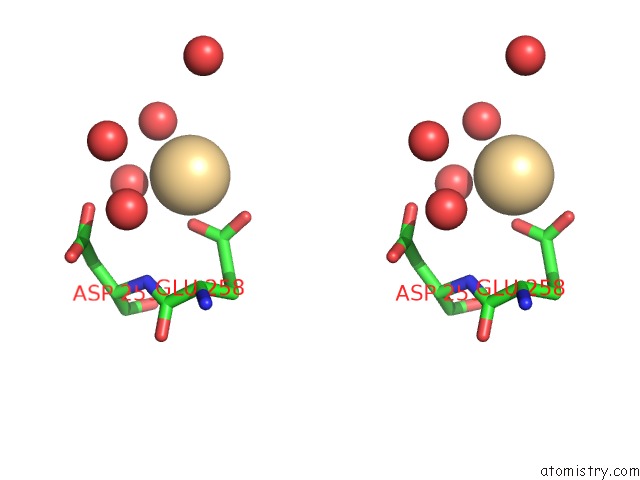
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cadmium with other atoms in the Cd binding
site number 1 of Human Gelsolin Domain 2 with A CD2+ Bound within 5.0Å range:
|
Cadmium binding site 2 out of 5 in 1kcq
Go back to
Cadmium binding site 2 out
of 5 in the Human Gelsolin Domain 2 with A CD2+ Bound
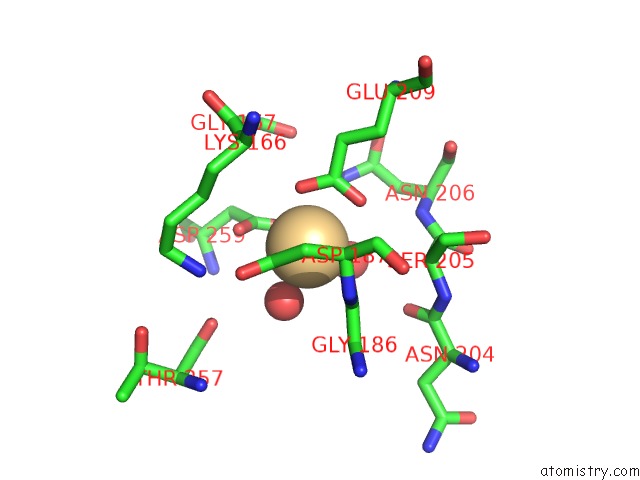
Mono view
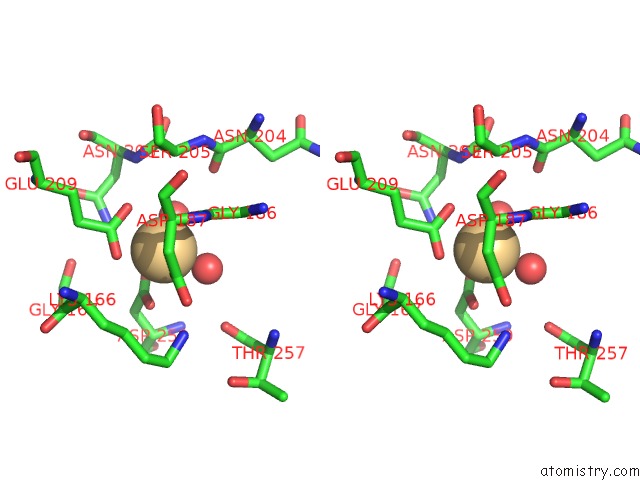
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cadmium with other atoms in the Cd binding
site number 2 of Human Gelsolin Domain 2 with A CD2+ Bound within 5.0Å range:
|
Cadmium binding site 3 out of 5 in 1kcq
Go back to
Cadmium binding site 3 out
of 5 in the Human Gelsolin Domain 2 with A CD2+ Bound
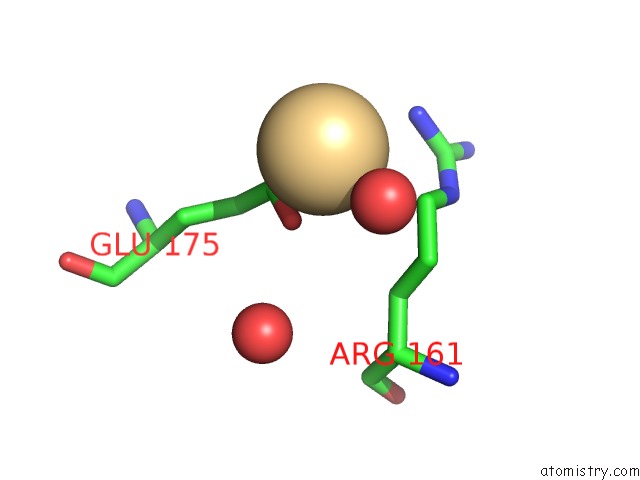
Mono view
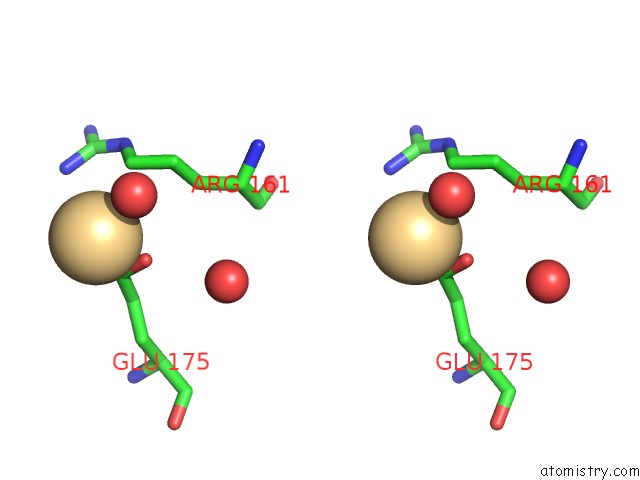
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cadmium with other atoms in the Cd binding
site number 3 of Human Gelsolin Domain 2 with A CD2+ Bound within 5.0Å range:
|
Cadmium binding site 4 out of 5 in 1kcq
Go back to
Cadmium binding site 4 out
of 5 in the Human Gelsolin Domain 2 with A CD2+ Bound
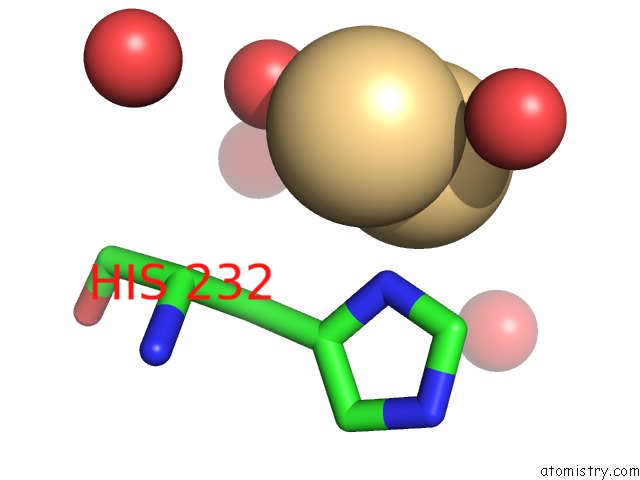
Mono view
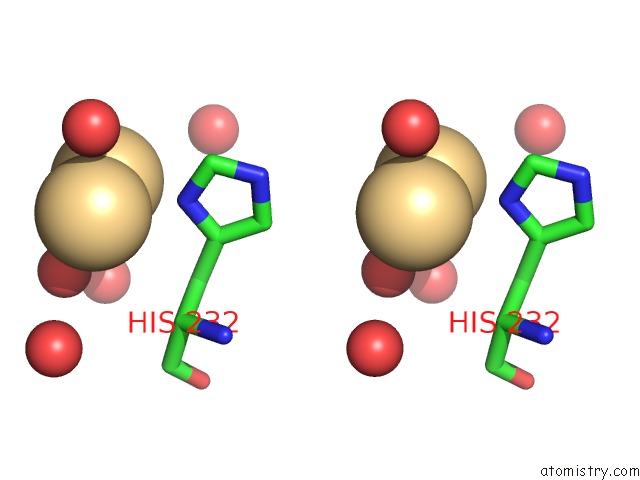
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cadmium with other atoms in the Cd binding
site number 4 of Human Gelsolin Domain 2 with A CD2+ Bound within 5.0Å range:
|
Cadmium binding site 5 out of 5 in 1kcq
Go back to
Cadmium binding site 5 out
of 5 in the Human Gelsolin Domain 2 with A CD2+ Bound
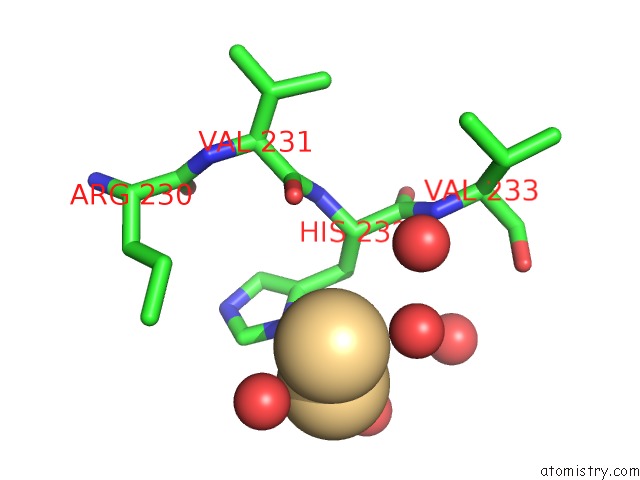
Mono view
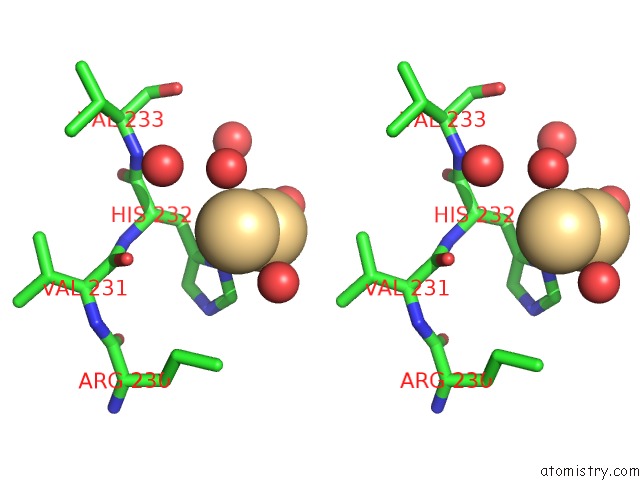
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cadmium with other atoms in the Cd binding
site number 5 of Human Gelsolin Domain 2 with A CD2+ Bound within 5.0Å range:
|
Reference:
S.L.Kazmirski,
R.L.Isaacson,
C.An,
A.Buckle,
C.M.Johnson,
V.Daggett,
A.R.Fersht.
Loss of A Metal-Binding Site in Gelsolin Leads to Familial Amyloidosis-Finnish Type. Nat.Struct.Biol. V. 9 112 2002.
ISSN: ISSN 1072-8368
PubMed: 11753432
DOI: 10.1038/NSB745
Page generated: Thu Jul 10 10:58:17 2025
ISSN: ISSN 1072-8368
PubMed: 11753432
DOI: 10.1038/NSB745
Last articles
F in 7MOGF in 7MOO
F in 7MML
F in 7MMI
F in 7MMK
F in 7MMJ
F in 7MMG
F in 7MMF
F in 7MMH
F in 7MMA